To compensate for the energy imbalance that comes with the application of renewable sources and availability of heat, energy storage has become an increasingly dominant research field in the academic world. For thermal energy storage, we can distinguish three fundamental methods. Storing heat by increasing the temperature of a storage material (sensible heat storage) and storing heat by changing the phase of a material from, for instance, solid to liquid (latent heat storage) are the most well-known. However, a third method, labelled as thermochemical or sorption heat storage, is seeing promising developments.
Sorption heat storage, how it works
When there is an abundance of thermal energy, this surplus can be used to heat up a sorption material (solid or liquid) that, when surpassing a certain temperature boundary, decomposes into a sorbent and sorbate. As the sorbent and sorbate appear in different states (solid/liquid and gaseous respectively) it’s easy to separate them physically. Once the need arises they can be recombined, releasing the ‘stored’ heat through and exothermal reaction with the sorption material again as its product [1] (see Figure 1 for a process illustration).
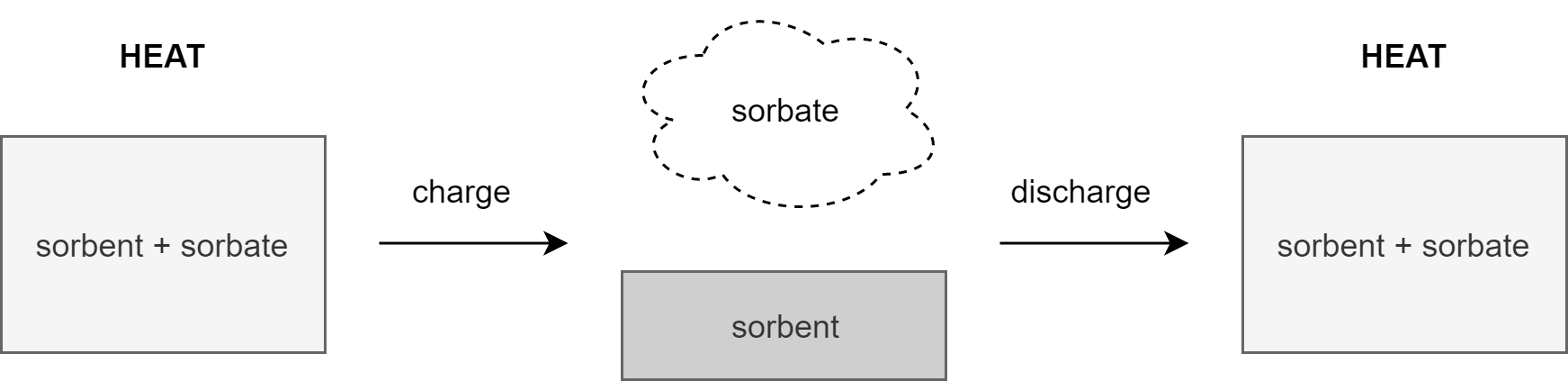
Figure 1: Sorption heat storage process
What distinguishes sorption heat storage from the conventional sensible and latent alternatives is that once the sorption material decomposes and its products are separated, there are no thermal losses. The sorbent and sorbate can be stored uninsulated as their embodied energy can only be released when they are mixed. The latter makes this particular storage application especially useful for seasonal applications, where the period between charging and discharging can span over several months [2].
To investigate the commercial application of sorption heat storage, researchers of the TU/e have designed and built a household-scale open sorption energy storage system based on the zeolite 13X/water reacting pair. The solid zeolite sorbent, applied as 3.5 mm spherical beads, was chosen as sorption material due to its high stability and storage density.
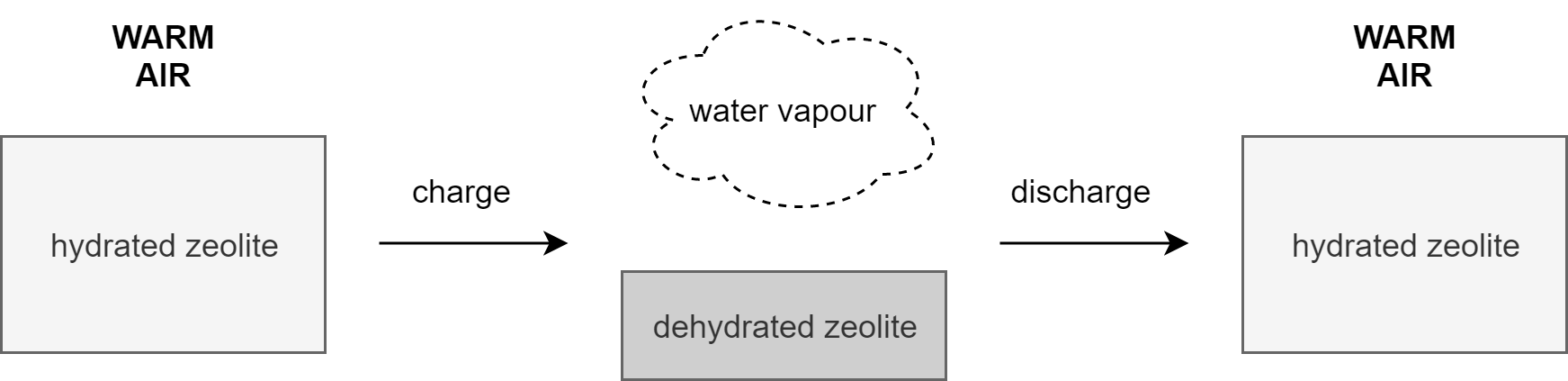
Figure 2: Sorption heat storage process for the zeolite 13X/water-configuration
The setup, which is described more elaboratively in [4], took into account the issues mentioned in existing literature. Instead of one big storage vessel, the design consists of four cuboid reactor segments of equal size, each containing 62.5 L of zeolite. Each segment has its own individual pipeline through which air is blown into the zeolite sorbent vessel. The air flow can, for each of the four reactors, be used to charge (dehydration) or discharge (hydration) the sorbent material. Triggering either of these processes is done by varying the air inlet temperature and humidity. The process of the zeolite 13X/water-configuration is shown in Figure 2 and a simplified schematic and picture of the test setup in Figure 3.
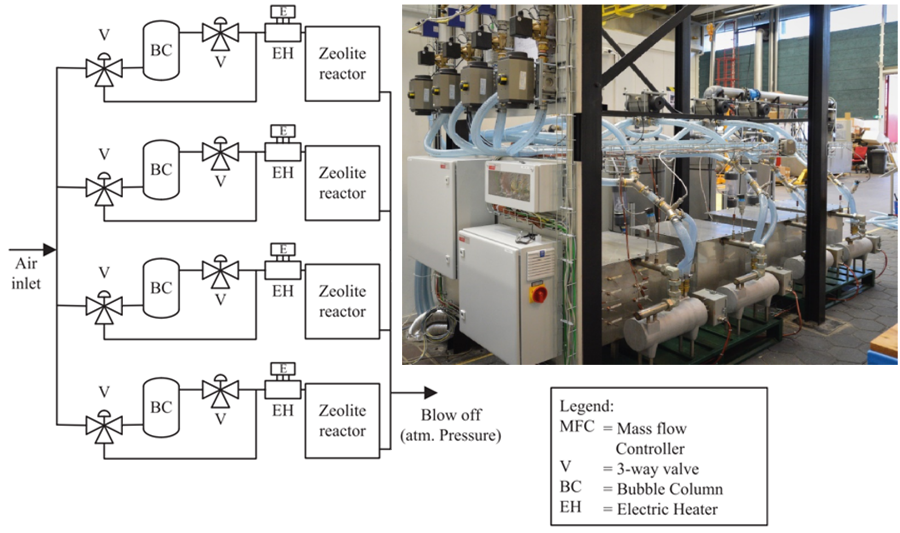
Figure 3: Simplified schematic overview (left) and picture of the of the experimental setup (right) [2].
Through testing, the setup has shown to be capable of storing 54 kWh of thermal energy. This energy could be discharged at 4.4 kW (max.) with a temperature difference of 24 degrees. When the reactor inlet temperature was pre-heated to 55°C, outlet temperatures of 75°C could be reached, indicating that with the addition of a heat-recovery system, domestic hot water production is also a possibility. Overall, a storage efficiency of 76% was achieved whist an increase to 91% is expected when the sensible heat of reactor components can be either recovered or contained through insulation.
Though the experimental setup provided promising results, there are still ways in which the system could improve. Most notably is the uniformity by which the air moves through the sorbent reactor. The uneven zeolite grain distribution for instance, was found to negatively impact the discharge stability and thus the overall system efficiency.
Written by C.W. Klok, you can download the article here.
Sources
[1] H. A. Zondag, Sorption Heat Storage. Elsevier Ltd., 2015.
[2] R. van Alebeek, L. Scapino, M. A. J. M. Beving, M. Gaeini, C. C. M. Rindt, and H. A. Zondag, “Investigation of a household-scale open sorption energy storage system based on the zeolite 13X/water reacting pair,†Appl. Therm. Eng., vol. 139, no. March, pp. 325–333, 2018.
[3] N. Yu, R. Z. Wang, and L. W. Wang, “Sorption thermal storage for solar energy,†Prog. Energy Combust. Sci., vol. 39, no. 5, pp. 489–514, 2013.
[4] M. Gaeini, M. R. Javed, H. Ouwerkerk, H. A. Zondag, and C. C. M. Rindt, “Realization of a 4kW thermochemical segmented reactor in household scale for seasonal heat storage,†Energy
